Introduction
The North America Hunter Syndrome Treatment Market is advancing rapidly with strong investment in biotechnology research, gene therapy development, and patient care infrastructure. Hunter syndrome, or Mucopolysaccharidosis type II (MPS II), is a rare, inherited lysosomal storage disorder caused by a deficiency of the iduronate-2-sulfatase (I2S) enzyme. This enzyme deficiency leads to the accumulation of glycosaminoglycans (GAGs) in tissues and organs, resulting in progressive organ dysfunction and neurological decline. In North America, rising awareness, enhanced diagnostic capabilities, and government-backed rare disease programs are accelerating the adoption of innovative therapies for this condition.
Market Size and Growth Projections
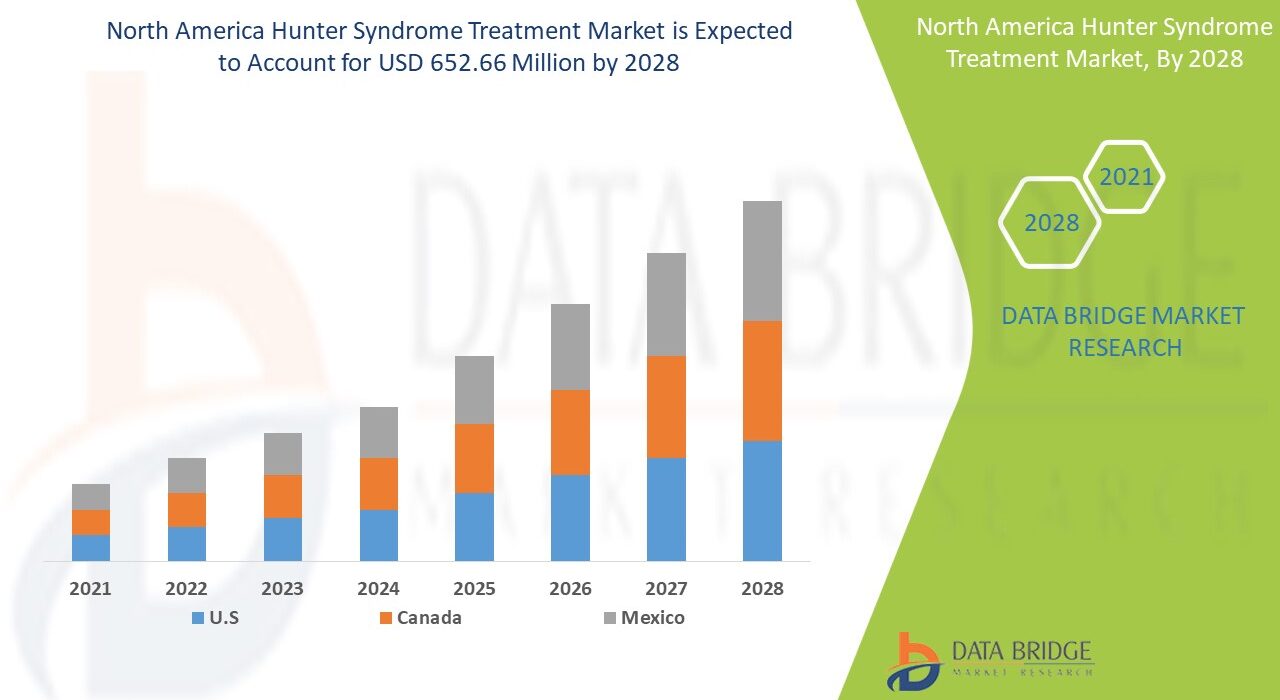
The North America Hunter Syndrome Treatment Market is expected to register a robust CAGR of XX% between 2024 and 2032, driven by growing clinical research, availability of enzyme replacement therapy (ERT), and breakthroughs in gene-based treatments. The United States dominates the regional market due to high healthcare expenditure, the presence of key pharmaceutical players, and strong regulatory support from the U.S. Food and Drug Administration (FDA) for orphan drugs.
Get More Details : https://www.databridgemarketresearch.com/reports/north-america-hunter-syndrome-treatment-market
Key Growth Factors
-
Increasing prevalence and early diagnosis of rare genetic disorders.
-
Continuous advancements in gene therapy platforms and biopharmaceutical innovations.
-
Availability of orphan drug incentives and funding support for R&D.
-
Expansion of specialized treatment centers and clinical trial networks.
-
Rising patient advocacy and awareness initiatives promoting early intervention.
Market Segmentation
By Treatment Type
-
Enzyme Replacement Therapy (ERT)
-
Gene Therapy
-
Hematopoietic Stem Cell Transplantation (HSCT)
-
Supportive and Symptomatic Treatment
By Route of Administration
-
Intravenous
-
Oral
-
Others
By End User
-
Hospitals and Clinics
-
Specialty Treatment Centers
-
Academic and Research Institutions
Regional Insights
-
United States: Leads the market owing to its advanced healthcare infrastructure, strong biotechnology sector, and FDA-backed rare disease framework. Multiple ongoing clinical trials in gene therapy are expanding treatment possibilities.
-
Canada: Emerging as a growing market due to improved access to orphan drugs, federal support for rare disease strategies, and increasing participation in international research collaborations.
Key Market Drivers
-
Growing Biopharmaceutical R&D: North America hosts several leading biotech firms focused on developing enzyme and gene therapies for MPS II.
-
Government Incentives: Policies such as the Orphan Drug Act in the U.S. provide regulatory and financial advantages for rare disease drug development.
-
Technological Advancements: Use of genomic sequencing and biomarker-based diagnosis is enhancing disease detection and management.
-
Collaborative Ecosystem: Strong partnerships between pharmaceutical companies, universities, and healthcare organizations accelerate innovation.
-
Patient Support Programs: Non-profit organizations and advocacy groups contribute to awareness, funding, and emotional support for affected families.
Market Challenges and Restraints
-
High cost of enzyme and gene therapies limiting accessibility for some patients.
-
Limited patient population posing challenges for large-scale clinical research.
-
Long and complex regulatory approval processes for advanced therapeutics.
-
Managing side effects and safety concerns in long-term therapy applications.
Competitive Landscape
The North America Hunter Syndrome Treatment Market is dominated by major global biopharmaceutical companies focusing on innovative therapeutic pipelines and expansion into gene therapy and regenerative medicine.
Key Companies:
-
Takeda Pharmaceutical Company Limited
-
Sanofi Genzyme
-
BioMarin Pharmaceutical Inc.
-
JCR Pharmaceuticals Co., Ltd.
-
Ultragenyx Pharmaceutical Inc.
-
Regenxbio Inc.
-
Denali Therapeutics Inc.
-
Orchard Therapeutics plc
Strategic Developments:
-
Expansion of clinical trials for AAV-based gene therapies in the U.S. and Canada.
-
Development of second-generation enzyme replacement therapies with improved biodistribution.
-
Collaborative research between biotech startups and academic institutions to enhance treatment outcomes.
-
Increased investment in patient-centered care and digital health integration for treatment monitoring.
Technological Innovations
-
CRISPR and Gene Editing Tools: Research on using CRISPR-Cas9 for long-term enzyme correction and disease modification.
-
Next-Generation Sequencing (NGS): Facilitates early and accurate diagnosis of MPS II.
-
Biomarker-Based Monitoring: Enables personalized therapy and progress tracking.
-
Nanocarrier Drug Delivery: Improves enzyme transport across the blood-brain barrier for neurological symptom management.
-
Artificial Intelligence (AI): Supports predictive modeling and treatment optimization for rare disease patients.
SWOT Analysis
| Strengths | Weaknesses | Opportunities | Threats |
|---|---|---|---|
| Advanced healthcare infrastructure and R&D ecosystem | High cost of gene therapy and enzyme treatment | Expansion of gene therapy clinical trials | Stringent regulatory and reimbursement barriers |
| Strong government and regulatory support for orphan drugs | Limited patient pool restricting scalability | Collaboration between biotech firms and healthcare providers | Competitive pricing pressure and patent expirations |
| Presence of leading pharmaceutical and biotech innovators | Long development and approval timelines | Increasing focus on precision medicine and rare disease funding | Adverse effects associated with new therapy modalities |
Future Market Outlook
The North America Hunter Syndrome Treatment Market is poised for continued growth as next-generation gene therapy, enzyme optimization, and cell-based therapies transition from research to commercialization. Government policies and private investments will further improve treatment affordability and access. Over the coming decade, breakthroughs in genetic medicine, coupled with AI-driven diagnostics and digital healthcare tools, will redefine how rare diseases like Hunter syndrome are managed.
Conclusion
The North America Hunter Syndrome Treatment Market represents a dynamic and transformative sector within the rare disease therapeutics landscape. Backed by technological innovation, strong clinical research, and a supportive policy environment, the region continues to lead the way in developing life-changing treatments for MPS II. As patient advocacy, funding, and genetic research continue to grow, the market will move closer to achieving curative and accessible solutions for individuals affected by Hunter syndrome.
Get More Reports :
https://www.databridgemarketresearch.com/reports/global-poultry-vaccines-market
https://www.databridgemarketresearch.com/reports/global-smart-manufacturing-market
https://www.databridgemarketresearch.com/reports/global-threat-intelligence-market
https://www.databridgemarketresearch.com/reports/global-cancer-cachexia-market
https://www.databridgemarketresearch.com/reports/global-craft-soda-market