Introduction
The Cardiac Safety Services Market plays a crucial role in the pharmaceutical and biotechnology sectors, providing essential support during clinical trials to ensure the cardiovascular safety of new drug candidates. These services encompass electrocardiogram (ECG) monitoring, cardiac imaging, risk assessment, and clinical data analysis aimed at detecting potential cardiotoxic effects. With growing regulatory scrutiny and the rising incidence of cardiovascular diseases, cardiac safety evaluation has become an indispensable component of drug development.
The integration of AI-driven ECG analysis, remote cardiac monitoring, and biostatistical modeling is transforming the landscape of cardiac safety services. Pharmaceutical companies are increasingly outsourcing these services to specialized vendors to reduce costs, streamline processes, and maintain regulatory compliance throughout all phases of clinical trials.
Get More Details : https://www.databridgemarketresearch.com/reports/global-cardiac-safety-services-market
Market Size and Growth Outlook
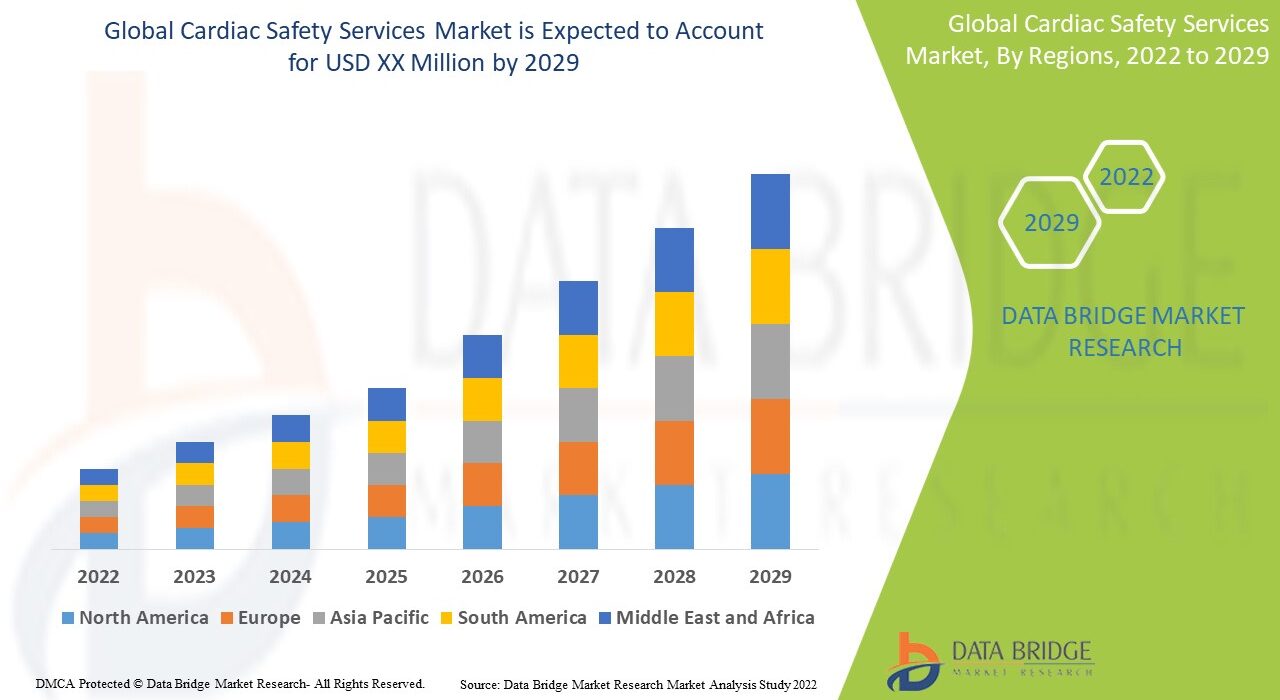
The global Cardiac Safety Services Market was valued at approximately USD 720 million in 2024 and is projected to reach USD 1.4 billion by 2032, growing at a CAGR of around 8.9% during the forecast period. The market expansion is driven by the increasing number of clinical trials, rising cardiovascular risk awareness, and advancements in digital cardiac safety technologies.
Market Segmentation
By Type of Service
-
ECG/Holter Measurement and Analysis Services – Includes centralized ECG analysis, continuous cardiac monitoring, and Holter testing.
-
Cardiac Imaging Services – Utilized for structural and functional heart assessment during clinical trials.
-
Thorough QT (TQT) Studies – Conducted to evaluate drug-induced QT interval prolongation.
-
Blood Pressure and Heart Rate Monitoring Services – Focused on detecting hemodynamic changes due to drug exposure.
-
Data Management and Biostatistics Services – Provide analysis, interpretation, and regulatory reporting.
By End User
-
Pharmaceutical Companies – Major consumers due to ongoing clinical trials and regulatory requirements.
-
Biotechnology Firms – Increasingly rely on outsourced cardiac safety services for new molecule testing.
-
Contract Research Organizations (CROs) – Act as intermediaries providing integrated cardiac safety solutions.
-
Academic and Research Institutes – Involved in early-stage clinical research and safety analysis.
By Phase of Trial
-
Preclinical
-
Phase I
-
Phase II
-
Phase III
-
Phase IV (Post-Marketing Surveillance)
Regional Insights
North America dominates the Cardiac Safety Services Market, driven by a large number of clinical trials, stringent FDA guidelines, and the presence of major pharmaceutical and CRO players. The U.S. leads in adoption of AI-based ECG monitoring systems and digital cardiac safety platforms.
Europe follows closely, with strong contributions from countries such as Germany, the UK, and France, supported by strict EMA regulatory frameworks and increased focus on patient-centric trials.
Asia-Pacific is witnessing rapid growth due to expanding clinical trial outsourcing in India, China, and South Korea. The region’s cost advantages, skilled workforce, and growing pharmaceutical R&D investments are propelling market expansion.
Latin America and Middle East & Africa are emerging markets, with gradual increases in local clinical research and partnerships with global CROs.
Key Market Drivers
-
Rising incidence of cardiovascular diseases increasing the focus on drug-induced cardiac safety.
-
Growing number of clinical trials requiring ECG and cardiac monitoring services.
-
Stringent regulatory requirements from agencies such as the FDA and EMA.
-
Adoption of AI, cloud computing, and digital monitoring tools to enhance cardiac safety analysis.
-
Outsourcing trend among pharmaceutical and biotech companies to reduce operational costs and improve data accuracy.
Market Challenges and Restraints
-
High cost associated with advanced cardiac imaging and digital monitoring systems.
-
Data privacy and regulatory compliance challenges, especially in cross-border trials.
-
Shortage of skilled professionals for ECG interpretation and cardiac data analytics.
-
Variability in regulatory standards across different regions.
Competitive Landscape
The Cardiac Safety Services Market is moderately consolidated, with key players offering comprehensive cardiac monitoring solutions through partnerships and technological advancements. Companies are investing in AI-based ECG analysis, cloud-based data management, and remote monitoring solutions to improve service efficiency and reliability.
Key Companies
-
Clario (formerly ERT)
-
IQVIA Holdings Inc.
-
Labcorp Drug Development
-
Bioclinica Inc.
-
Medpace Holdings, Inc.
-
SGS SA
-
Banook Group
-
Celerion
-
Biotrial
-
Richmond Pharmacology
-
Veristat, LLC
-
PhysioStim
Recent Developments
-
Integration of AI and machine learning algorithms for enhanced ECG interpretation.
-
Expansion of remote cardiac monitoring systems for decentralized clinical trials.
-
Strategic mergers and acquisitions among CROs to expand service portfolios.
-
Development of cloud-based cardiac data management platforms to improve data accuracy and accessibility.
SWOT Analysis
| Strengths | Weaknesses |
|---|---|
| Essential component of regulatory compliance in drug development | High cost of advanced testing and data systems |
| Strong outsourcing demand from pharmaceutical and biotech firms | Dependence on qualified cardiac specialists |
| Integration with AI and digital health platforms | Limited standardization across global regions |
| Opportunities | Threats |
|---|---|
| Growing clinical trials in emerging markets | Data security and privacy concerns |
| Adoption of AI-driven ECG and predictive analytics | Regulatory complexities and delays |
| Expansion into decentralized and virtual trials | Competition from in-house testing capabilities |
Emerging Trends
-
Shift toward remote and decentralized clinical trials with real-time cardiac monitoring.
-
AI-enabled ECG platforms providing faster and more accurate arrhythmia detection.
-
Growing adoption of wearable devices for continuous cardiac safety tracking.
-
Cloud integration and digital data management improving clinical trial transparency.
-
Emphasis on personalized cardiac risk assessment in precision medicine.
Future Market Outlook
The Cardiac Safety Services Market is poised for significant expansion as the pharmaceutical industry accelerates new drug development and digital transformation. The convergence of AI, telemedicine, and advanced ECG technologies will redefine cardiac safety monitoring, making it more predictive and efficient. Outsourcing partnerships between pharmaceutical companies and CROs will continue to grow, driven by the need for regulatory compliance and cost-effective service models.
Conclusion
The Cardiac Safety Services Market serves as a cornerstone of modern clinical research, ensuring that new therapeutics meet rigorous safety standards before reaching the market. As cardiovascular health continues to be a global priority, the integration of AI-driven analytics, wearable monitoring technologies, and cloud-based platforms will revolutionize how cardiac safety is assessed. The market’s future lies in automation, precision, and real-time data intelligence, enabling faster, safer, and more effective drug development worldwide.
Get More Reports :
https://www.databridgemarketresearch.com/reports/global-ip-vpn-web-hosting-service-market
https://www.databridgemarketresearch.com/reports/global-paints-coatings-market
https://www.databridgemarketresearch.com/reports/global-esim-market
https://www.databridgemarketresearch.com/reports/global-premium-wine-market
https://www.databridgemarketresearch.com/reports/global-antibody-drug-conjugates-market