Introduction
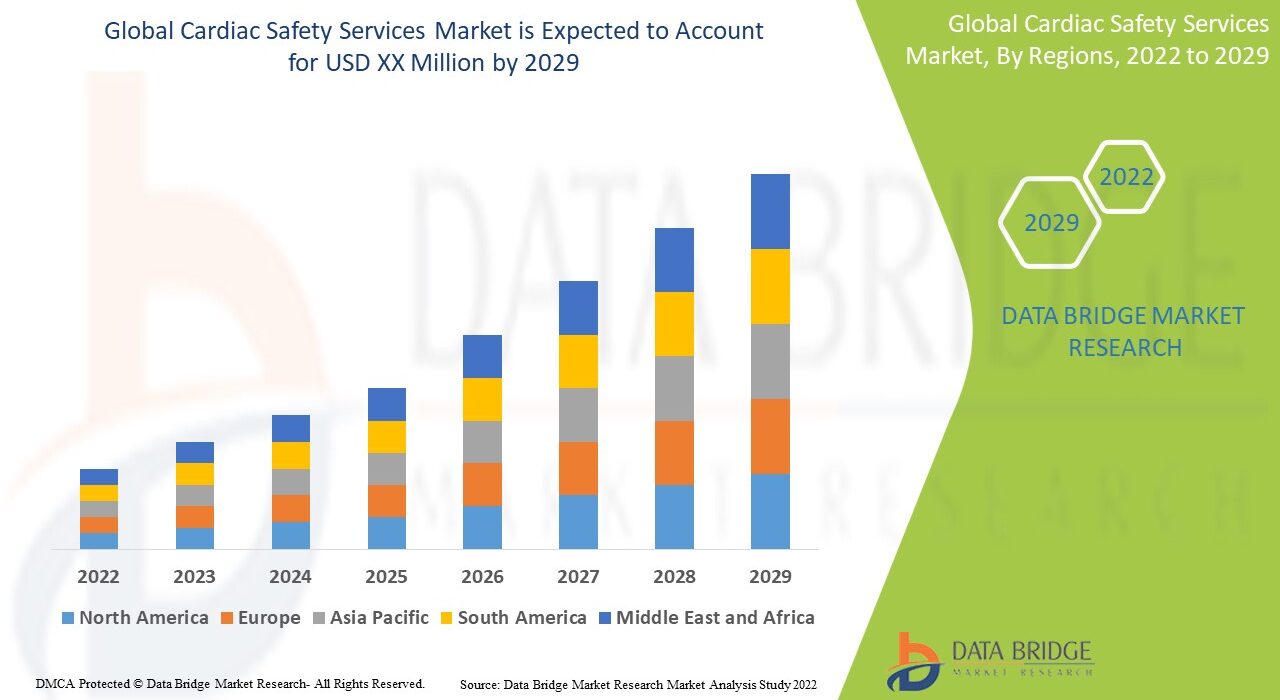
The Cardiac Safety Services Market focuses on providing specialized testing, monitoring, and analytical solutions that evaluate the cardiovascular safety profile of new drugs and medical devices. These services are critical in clinical trials to ensure that therapeutics do not cause adverse cardiac effects, thereby maintaining patient safety and regulatory compliance.
As cardiovascular complications remain a leading cause of clinical trial failure, pharmaceutical and biotechnology companies increasingly rely on cardiac safety service providers for end-to-end solutions. Technological advancements in electrocardiogram (ECG) analysis, imaging, and predictive modeling are driving greater accuracy and efficiency in cardiac monitoring.
Get More Details : https://www.databridgemarketresearch.com/reports/global-cardiac-safety-services-market
Market Size and Growth Projections
The global Cardiac Safety Services Market was valued at USD XX billion in 2024 and is projected to reach USD XX billion by 2032, expanding at a CAGR of XX% during the forecast period. Growth is propelled by the rising volume of clinical trials, increasing cardiovascular disease prevalence, and growing emphasis on patient safety regulations across pharmaceutical development.
Key Growth Factors:
-
Rising number of drug discovery and clinical research programs.
-
Increasing global prevalence of cardiovascular diseases.
-
Expansion of outsourcing trends among pharmaceutical and biotech companies.
-
Growing adoption of digital ECG and centralized cardiac safety analysis.
-
Strict regulatory frameworks for cardiac monitoring during drug development.
Market Segmentation
By Type
-
ECG/Holter Measurement
-
Blood Pressure Measurement
-
Thorough QT Studies (TQT)
-
Cardiovascular Imaging
-
Other Cardiac Safety Assessments
By Service Type
-
Preclinical Services
-
Clinical Services
-
Post-Market Surveillance Services
By End User
-
Pharmaceutical Companies
-
Biotechnology Firms
-
Contract Research Organizations (CROs)
-
Academic and Research Institutes
By Therapeutic Area
-
Oncology
-
Infectious Diseases
-
Neurology
-
Cardiovascular Diseases
-
Others
Regional Insights
North America:
Dominates the global market due to the presence of major CROs, a large number of clinical trials, and strong regulatory oversight by the FDA. The U.S. remains a key hub for drug safety monitoring and pharmacovigilance innovation.
Europe:
Shows steady growth driven by an established pharmaceutical industry, regulatory harmonization across the EU, and advancements in e-clinical trial management systems. The UK, Germany, and Switzerland lead regional adoption.
Asia-Pacific:
Expected to experience the fastest growth, fueled by the expansion of clinical trial outsourcing, growing biotech investment, and rising demand for cost-efficient cardiac safety monitoring solutions in India, China, and South Korea.
Latin America:
Emerging market driven by increased clinical trial activity, particularly in Brazil and Mexico, supported by government initiatives to promote pharmaceutical research.
Middle East & Africa:
Gradual growth due to expanding healthcare infrastructure and increasing participation in global clinical research networks, particularly in South Africa and the UAE.
Key Market Drivers
-
Rising Clinical Trial Volume: Increasing number of global drug trials requiring cardiac safety assessments.
-
Regulatory Compliance: Stringent safety standards by the FDA, EMA, and other agencies.
-
Technological Innovation: Adoption of AI-driven ECG analytics and remote monitoring tools.
-
Pharmaceutical Outsourcing Trends: Growing preference for specialized cardiac safety CROs.
-
Rising Cardiovascular Disease Burden: Increasing patient populations requiring safety testing for heart-related side effects.
Market Challenges and Restraints
-
High Cost of Cardiac Safety Testing: Advanced monitoring and imaging services can be expensive.
-
Data Management Complexity: Handling vast ECG and imaging data from multiple trial sites.
-
Regulatory Delays: Extended approval timelines due to stringent safety validation.
-
Shortage of Skilled Professionals: Need for trained cardiac physiologists and ECG experts.
-
Limited Access in Developing Regions: Lack of infrastructure for advanced safety monitoring.
Competitive Landscape
The Cardiac Safety Services Market is moderately consolidated, with leading players offering integrated preclinical and clinical safety solutions. Companies are focusing on advanced analytics, global expansion, and digital transformation of monitoring systems.
Key Companies:
-
Bioclinica, Inc. (Clario)
-
IQVIA Holdings Inc.
-
Laboratory Corporation of America Holdings (Labcorp)
-
Charles River Laboratories International, Inc.
-
Medpace Holdings, Inc.
-
ERT (eResearch Technology, Inc.)
-
Celerion, Inc.
-
SGS SA
-
Banook Group
-
Certara, Inc.
-
Biomedical Systems Corporation
Strategic Developments:
-
Integration of cloud-based ECG data platforms for real-time monitoring.
-
Expansion of service networks in Asia-Pacific and Latin America.
-
Strategic partnerships between CROs and pharmaceutical developers.
-
AI-based predictive cardiac safety analytics for early risk detection.
-
Development of wearable cardiac monitoring devices for decentralized trials.
Technological Innovations
-
AI-Driven ECG Analysis: Automated detection of cardiac anomalies in real-time.
-
Wearable Monitoring Devices: Continuous and remote cardiac safety tracking.
-
Cloud-Based Data Platforms: Centralized and secure cardiac data management for global trials.
-
Integrated Risk Prediction Models: Early identification of cardiotoxicity in drug candidates.
-
Digital Twin Technology: Simulated patient modeling to predict cardiac safety outcomes.
SWOT Analysis
| Strengths | Weaknesses | Opportunities | Threats |
|---|---|---|---|
| Expertise in regulatory-compliant cardiac testing | High cost of advanced cardiac safety systems | Expansion of clinical trials in emerging markets | Increasing competition among CROs |
| Integration of AI and digital ECG systems | Limited awareness in developing regions | Rising demand for decentralized clinical trials | Regulatory complexities across countries |
| Strong partnerships with pharma and biotech firms | Dependence on high-end equipment | Technological innovation in real-time data analytics | Data privacy and cybersecurity risks |
Future Market Outlook
The Cardiac Safety Services Market is poised for sustained growth as global clinical trials continue to expand and regulatory agencies tighten safety monitoring standards. The future of cardiac safety lies in AI-powered analytics, cloud-based platforms, and wearable technologies that enable real-time data acquisition and predictive insights.
As personalized medicine and biologics enter mainstream therapeutics, cardiac safety will become integral to early-phase testing and risk mitigation. Partnerships between CROs, tech providers, and pharmaceutical firms will drive cost efficiency, data transparency, and innovation in the coming decade.
Conclusion
The Cardiac Safety Services Market plays a pivotal role in ensuring patient safety and regulatory compliance throughout the drug development lifecycle. With rapid technological advancements, the integration of AI, remote monitoring, and digital analytics is transforming how cardiac safety is assessed and managed. The future market landscape will favor organizations that combine innovation, clinical expertise, and global reach to deliver efficient, accurate, and patient-centric cardiac safety solutions.
Get More Reports :
https://www.databridgemarketresearch.com/reports/global-meditation-market
https://www.databridgemarketresearch.com/reports/global-shampoo-market
https://www.databridgemarketresearch.com/reports/global-lidar-market
https://www.databridgemarketresearch.com/reports/global-biomaterials-market
https://www.databridgemarketresearch.com/reports/global-dermatology-devices-market