Introduction
The Next-Generation Biomanufacturing Market represents a transformative shift in the production of biologics, vaccines, cell and gene therapies, and biosimilars. It encompasses advanced technologies, digital process control, and automation designed to increase efficiency, reduce costs, and improve product quality. The market includes continuous bioprocessing systems, single-use bioreactors, modular facilities, and digital twins that are redefining how biopharmaceuticals are developed and produced.
The importance of this market lies in its ability to enhance agility, flexibility, and productivity across the biopharmaceutical industry. It supports the growing need for scalable production of complex molecules and personalized treatments, aligning with the global push toward efficiency and precision in healthcare manufacturing.
Learn how the Next-Generation Biomanufacturing Market is evolving—insights, trends, and opportunities await. Download report: https://www.databridgemarketresearch.com/reports/global-next-generation-biomanufacturing-market
The Evolution
The evolution of biomanufacturing began in the 1980s with the introduction of recombinant DNA technology and monoclonal antibody production. Early processes relied heavily on stainless steel bioreactors, large-scale facilities, and batch-based systems. These methods were time-consuming, capital-intensive, and limited in flexibility.
By the early 2000s, the biopharmaceutical industry began transitioning toward single-use systems (SUS) and disposable bioreactors, which offered greater operational flexibility and reduced cleaning validation times. This transition marked the foundation of next-generation biomanufacturing.
The introduction of continuous bioprocessing, process analytical technology (PAT), and digital process control reshaped the production landscape. Advances in automation, robotics, and artificial intelligence now enable real-time monitoring, predictive maintenance, and optimization of manufacturing workflows.
The COVID-19 pandemic further accelerated innovation, pushing manufacturers to adopt rapid, scalable, and decentralized production models. Modular biomanufacturing facilities emerged, allowing localized production of vaccines and biologics with shorter construction timelines.
Today, next-generation biomanufacturing integrates machine learning, data analytics, and modular automation to achieve end-to-end process efficiency, sustainability, and cost-effectiveness across the global biopharmaceutical supply chain.
Market Trends
1. Shift Toward Continuous Manufacturing
Continuous bioprocessing is gaining traction as a method to improve efficiency and product consistency. Unlike traditional batch processing, continuous systems minimize downtime and enable real-time quality control.
2. Adoption of Single-Use Technologies (SUTs)
The demand for single-use bioreactors and filtration systems is increasing due to reduced cleaning requirements, faster turnaround times, and lower cross-contamination risks. These technologies are becoming standard for both clinical and commercial-scale production.
3. Integration of Digitalization and Automation
Digital twins, AI-driven analytics, and automated control systems are enabling predictive maintenance, process optimization, and reduced human error. Smart biomanufacturing platforms are improving yield and reproducibility.
4. Rising Popularity of Modular Biomanufacturing Facilities
Companies are investing in modular, pre-fabricated facilities that can be rapidly deployed and customized. This trend enhances manufacturing flexibility and allows production to be scaled closer to demand centers.
5. Sustainability and Energy Efficiency
Environmental sustainability is a growing focus. Next-generation systems consume less water, energy, and cleaning chemicals, aligning with global green manufacturing initiatives.
6. Expansion of Cell and Gene Therapy Manufacturing
The growing pipeline of cell and gene therapies is creating demand for advanced production platforms capable of handling small-batch, high-complexity biologics. Closed and automated systems are becoming essential in this segment.
Challenges
1. High Capital Investment and Implementation Costs
Despite long-term savings, initial investments in digital infrastructure, automation, and continuous manufacturing systems are substantial, particularly for small and mid-sized biotech firms.
2. Regulatory Complexity
The adoption of new bioprocessing models faces regulatory uncertainty. Existing frameworks are often based on batch processes, and harmonizing guidelines for continuous manufacturing remains a challenge.
3. Technical Skill Gaps
The implementation of next-generation technologies requires specialized expertise in automation, data analytics, and process design. Workforce training and skill development are critical barriers to widespread adoption.
4. Supply Chain and Material Standardization Issues
Inconsistent supply and quality of single-use components can disrupt operations. The lack of universal standards in connectors, sensors, and disposable components affects interoperability.
5. Data Integration and Cybersecurity Risks
Increased digitalization raises cybersecurity concerns and challenges in integrating legacy systems with new digital platforms. Data protection and integrity are vital for regulatory compliance.
6. Scaling Limitations for Complex Therapies
Manufacturing personalized treatments, such as autologous cell therapies, presents scalability challenges. The need for closed, automated systems is essential to maintain consistency across batches.
Market Scope
Segmentation by Type
-
Continuous Bioprocessing Systems
-
Single-Use Bioprocessing Systems
-
Hybrid Manufacturing Systems
-
Modular Biomanufacturing Platforms
Segmentation by Technology
-
Upstream Bioprocessing
-
Downstream Bioprocessing
-
Process Analytics and Automation
-
Digital Twin and Data-Driven Control Systems
Segmentation by Application
-
Monoclonal Antibody Production
-
Vaccine Manufacturing
-
Cell and Gene Therapy
-
Recombinant Protein Production
-
Biosimilar Development
Segmentation by End User
-
Biopharmaceutical Companies
-
Contract Development and Manufacturing Organizations (CDMOs)
-
Academic and Research Institutions
-
Clinical Laboratories
Regional Analysis
North America
North America holds the largest share of the next-generation biomanufacturing market due to the strong presence of leading biopharma companies and early adoption of digital and single-use technologies. The U.S. remains a hub for innovation, supported by FDA regulatory initiatives promoting continuous manufacturing.
Europe
Europe follows closely, with major investments in sustainable and modular manufacturing. The UK, Germany, and Switzerland are key contributors, focusing on bioprocess automation and regulatory alignment to enhance production capacity.
Asia-Pacific
Asia-Pacific is the fastest-growing region, driven by expanding biopharmaceutical production capabilities in China, India, South Korea, and Japan. Governments are promoting domestic biologics manufacturing through funding and technology partnerships.
Latin America
The market in Latin America is expanding as countries such as Brazil and Mexico strengthen their biomanufacturing infrastructure to meet local vaccine and biologics demand.
Middle East & Africa
Growth in this region is driven by efforts to establish self-sufficient biopharmaceutical production networks. Investments in modular facilities are increasing to improve access to essential medicines and vaccines.
Market Size and Factors Driving Growth
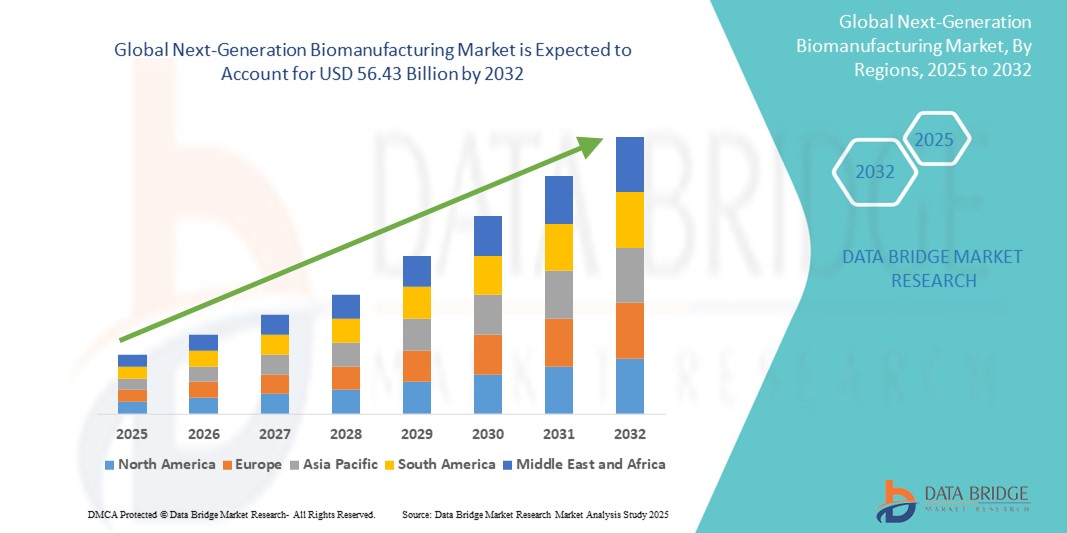
- The global next-generation biomanufacturing market size was valued at USD 26.61 billion in 2024 and is expected to reach USD 56.43 billion by 2032, at a CAGR of 9.85% during the forecast period
Major Growth Drivers
1. Growing Biologics and Biosimilar Demand
The expanding global pipeline of biologics, monoclonal antibodies, and biosimilars requires scalable, efficient, and cost-effective production technologies.
2. Technological Advancements in Automation and AI
Artificial intelligence, machine learning, and data analytics enable real-time monitoring and predictive control, enhancing process efficiency and product quality.
3. Rising Focus on Personalized Medicine
Next-generation biomanufacturing supports small-batch production, which is essential for personalized therapies such as CAR-T cells and gene-modified treatments.
4. Expansion of Contract Manufacturing Organizations (CMOs and CDMOs)
Pharmaceutical companies are outsourcing production to specialized CDMOs equipped with next-generation bioprocessing capabilities to reduce capital costs and increase flexibility.
5. Increasing Government and Industry Collaboration
Governments and regulatory bodies are supporting the transition toward modernized production systems through policy incentives, public-private partnerships, and funding initiatives.
6. Sustainability and Operational Efficiency
Adoption of energy-efficient and low-waste bioprocesses aligns with corporate sustainability goals, reducing the environmental impact of pharmaceutical production.
Opportunities in Emerging Regions
Emerging markets in Asia-Pacific and the Middle East offer vast potential due to rising healthcare demand and government initiatives promoting local biologics manufacturing. Partnerships between global and regional players are fostering technology transfer and infrastructure development.
Automation, single-use systems, and modular production units are being deployed to establish flexible manufacturing capacity in these regions, supporting faster response to pandemics and public health needs.
Conclusion
The next-generation biomanufacturing market represents the future of global biologics production. The transition from traditional batch systems to continuous, automated, and data-driven processes is creating a more agile and sustainable manufacturing environment.
With a projected growth from USD 20 billion in 2024 to USD 65 billion by 2035, the market is set for significant expansion. The integration of artificial intelligence, digital twins, and single-use technologies will define the next phase of industrial innovation.
Sustainability, flexibility, and precision will remain central to future development. Stakeholders focusing on digital transformation, workforce training, and collaborative R&D will gain a competitive edge. The market’s evolution aligns with global healthcare trends, driving faster, safer, and more efficient biologics production to meet the world’s growing medical needs.
FAQs
1. What is the current size of the next-generation biomanufacturing market?
The market is valued at approximately USD 20 billion in 2024.
2. What is the expected market size by 2035?
The global next-generation biomanufacturing market is projected to reach USD 65 billion by 2035, growing at a CAGR of 11.2%.
3. What are the main technologies driving market growth?
Continuous manufacturing, single-use systems, automation, and digital process control are the key technological drivers.
4. Which region holds the largest market share?
North America leads the market due to strong biopharmaceutical infrastructure and advanced R&D capabilities.
5. Which region is expected to experience the fastest growth?
Asia-Pacific is the fastest-growing region, supported by large-scale investments in biomanufacturing infrastructure and government initiatives.
6. What are the primary challenges in this market?
Challenges include high capital costs, regulatory complexity, technical skill shortages, and data integration risks.
7. What opportunities exist for new entrants?
Opportunities include partnerships with CDMOs, investment in modular facilities, and adoption of AI-driven process optimization tools in emerging markets.
8. What industries are the major end users of next-generation biomanufacturing?
Biopharmaceutical companies, contract manufacturing organizations, and academic research institutions are the key end users.
Browse More Reports:
Europe RF Over Fiber Market
North America RF Over Fiber Market
Asia-Pacific RF Over Fiber Market
Middle East and Africa RF Over Fiber Market
Europe Rotomolding Products Market
Asia-Pacific Rotomolding Products Market
North America Rotomolding Products Market
Europe Satellite Transponder Market
North America Satellite Transponder Market
Asia-Pacific Semiconductor IP Market
Europe Semiconductor IP Market
Middle East and Africa Semiconductor IP Market
North America Semiconductor IP Market
China Silicon Anode Material Battery Market
Japan Silicon Anode Material Battery Market
About Data Bridge Market Research:
An absolute way to forecast what the future holds is to comprehend the trend today!
Data Bridge Market Research set forth itself as an unconventional and neoteric market research and consulting firm with an unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process. Data Bridge is an aftermath of sheer wisdom and experience which was formulated and framed in the year 2015 in Pune.
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC : +653 1251 975
Email:- corporatesales@databridgemarketresearch.comNext-Generation Biomanufacturing Market Size